Within Vault, an organization may use the QMS application in a Quality Vault to ensure that a change to a product or system is introduced in a controlled and coordinated manner. The same organization may use the Registrations application in a RIM Vault determine the impacted markets for that change and manage the filing updates and approvals for relevant health authorities. In such a case, using separate systems can result in duplicate data entry and a lack of synchronization, which prolongs the process and results in changes that occur without a proper understanding of regulatory impact.
This feature enables organizations using both a Quality Vault and a RIM Vault to share data about change events that have regulatory impact. The Quality-RIM Connection automates document and data sharing between these two applications, supporting change control initiation, regulatory assessment, and close out.
Note: This connection and its various integration capabilities must be configured by an Admin.
About the Quality-RIM Connection
The Quality to RIM Connection helps to solve several industry challenges that exist for change events, including:
- Regulatory Impact Assessments: When users create change controls in a Quality Vault, they define the scope of the change by creating Regulatory Change Item records. Each Regulatory Change Item record identifies a type of change, such as a manufacturing equipment change, and the item being changed (for example, a Wonderdrug Tablet 40mg). A QMS change control can include multiple Regulatory Change Items. At the appropriate point in the change control process, the connection automatically creates Change Item records in the Registrations Vault–-one for each Regulatory Change Item in the QMS Change Control. Regulatory organizes Change Items into Events based on their filing strategy and conducts an Impact Assessment, producing Activity Change Item records representing a type of change, such as a manufacturing equipment change, to an item (for example, a Wonderdrug Tablet 40mg) in a specific market, like the United States. In order to reflect the Regulatory Impact Assessment results in your Quality Vault, the connection automatically creates Regulatory Activity Item records in QMS for each Activity Change Item in Registrations. The Regulatory Activity Items are linked to the source change control record, and Quality users working on the change control can see the status of the regulatory activities in real time.
- Filing Updates: When the change control is approved for implementation in QMS, the connection automatically alerts country users in Registrations that they can begin their filings to get the change approved for all countries and relevant health authorities.
- Product Transfer: When a Product, Product Family, or Product Variant record is created or edited in a RIM Vault, the connection creates or updates the record in the Quality Vault. This data can then be associated with Change Controls.
- Document Exchange: A source Quality or RIM Vault document is transferred with its metadata to the target Vault as a CrossLink. The connection maintains the CrossLink and metadata as the source document undergoes updates and re-enters the Steady state, or enters the Superseded and Obsolete states.
Note: As of the 25R1 release, the Quality-RIM Connection’s Variation Management (Change Management) feature is superseded by the Enhanced Change Control feature. Veeva customers should consider using the connection’s Enhanced Change Control feature instead.
How the Quality to RIM Connection Works
The Quality-RIM Connection includes the features below.
In most cases, these integrations can run independently or together. The Product Transfer integration must be enabled in order to use Enhanced Change Control.
Enhanced Change Control
The connection’s Enhanced Change Control feature allows Vault to recognize a many-to-many relationship between Quality Event records in RIM Vaults and Change Control records in Quality Vaults, which provides added flexibility for users completing assessments, determining an appropriate filing strategy, completing submissions, and tracking partial health authority decisions.
This connection includes two QMS standard objects and lifecycles, Regulatory Change Item and Regulatory Activity Item, that interact with two standard RIM objects, Change Item and Activity Change Item.
- When QMS team members create Change Controls in their Quality Vault that require regulatory assessments, the Change Controls are tied to the Product, Product Family, and Product Variant records through the manual creation of Regulatory Change Item records.
- The connection then creates Change Items in the RIM Vault, and Regulatory team members can associate the appropriate Event records in RIM. When additionally configured, RIM Vaults can either:
- Create Event records with related Change Items for each Quality Change Control, or
- Create separate Event records with related Change Items for each combination of Quality Change Control and Product Family records.
- During regulatory assessments, Regulatory users in the RIM Vault can notify the Quality team that corrections are needed on a Change Control. Regulatory users can update a Change Item in an In Assessment state, populating the Correction Required field and setting the state to Requires Correction. The connection automatically updates the corresponding Regulatory Change Item in the Quality Vault and notifies the Quality Team that updates are required. Once the Quality Team has made the necessary changes to the Change Control, they can update the Regulatory Change Item by populating the field Correction Details and setting the state to Corrected. Should the Quality team need to notify the Regulatory team a correction is needed while the record is in In Assessment state, they can change the Regulatory Change Item state to Corrected and enter details of the change in the Correction Details field.
- This two-step process does not impact Activity Change Item or Regulatory Activity Item records.
Regulatory team members can also bundle and split Activity Change Items to support their business needs. In turn, the connection creates Regulatory Activity Items in Quality Vaults, which provides Quality teams the status of each Product in each impacted market. Once Activities are dispositioned, submissions are complete, and health authority decisions are received, the connection updates the status of the Activity Change Items in RIM and the Regulatory Activity Items in QMS. Quality teams can provide individual assessments on each item affected by a Change Control and request reassessments of individual items if the scope of the Change Control changes. Regulatory teams can create an appropriate filing strategy based on the impact of each change in a Change Control record.
Document Exchange
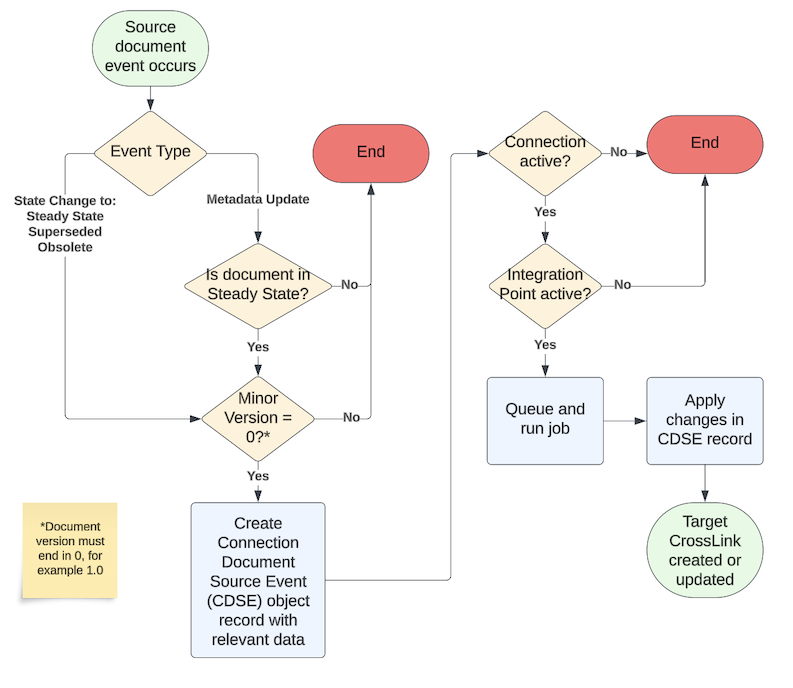
The connection’s feature for Document Exchange enables Vault to create and maintain document CrossLinks and metadata across your Quality and RIM Vaults. The document types and fields Vault transfers are determined and mapped by an Admin when configuring the feature. As such, a document in either Vault can be considered to be the source (outbound) or target (inbound) document.
When, for example, a source Quality document reaches its Steady state, Vault creates a CrossLink document in the target RIM Vault and populates metadata accordingly. Once created, Vault maintains the target CrossLink when the source document:
- Undergoes a metadata update while in the Steady state.
- Re-enters the Steady state after up-versioning.
- Enters the Superseded or Obsolete state.
See the use case below and the feature’s limitations for more information.
The diagram below provides a basic overview of this process, as well as the various criteria which must be met in order for a CrossLinked document to be created and maintained in a target Vault.
Document Exchange Use Case
VeePharm classifies master batch records in Quality under the Operations > Master Batch Record document subtype, and in RIM under the Quality > Regional Information > Batch Records (Master/Executed) classification.
These documents’ respective lifecycles map state types to states as follows:
| State Type | Quality State | RIM State |
|---|---|---|
| Steady State | Effective | Approved |
| Superseded State | Superseded | Superseded |
| Obsolete State | Obsolete | Obsolete |
The table below illustrates Vault actions and outcomes for a single Master Batch Record document as it undergoes various document events throughout its lifecycle.
| Quality Master Batch Record document event | RIM Batch Record (Master/Executed) CrossLink result |
|---|---|
| Quality draft document v0.1 routed for approval, entering the Approved state v1.0. | None. Quality’s Approved state is not the Steady state, therefore a CrossLink is not created. |
| Quality document v1.0 moved to the Effective (Steady) state. | RIM CrossLink v1.0 created in the Approved (Steady) state. |
| Quality document v1.1 created and routed for approval. It enters the Approved state (v2.0), then the Effective (Steady) state. Document v1.0 set to the Superseded state. | RIM CrossLink updated to v2.0, remaining in the Approved (Steady) state. CrossLink v1.0 set to the Superseded state. |
| Quality document v2.0 Country document field updated from “United States” only to “United States” and “Puerto Rico”. | RIM CrossLink v2.0 Country document field updated from “United States” only to “United States” and “Puerto Rico”. |
| Quality document v2.0 moved to the Obsolete state. | RIM CrossLink v2.0 moved to the Obsolete state. |
The examples above mostly follow a positive flow, in that document events are encountered and processed as expected. Below are some possible scenarios in which a new or updated Quality Master Batch Record produces different results in RIM.
| Quality Master Batch Record document event | Scenario | RIM Batch Record (Master/Executed) CrossLink result |
|---|---|---|
| A VeePharm Admin is migrating finalized batch records from a local drive, creating a Quality document v3.0 in the Effective (Steady) state. | The document exists only in the Steady state. It does not enter the Steady state from another state during the migration. | None. The integration does not create target CrossLinks for source documents created in the Steady state. |
| Quality document v1.0 up-versioned in quick succession to v2.0, then v3.0. Both versions enter the Effective (Steady) state. | Rapid transition to v3.0 occurs before the connection job processes the transition to v2.0. | RIM CrossLink v1.0 up-versioned to v3.0 in the Approved (Steady) state. Version history reflects versions 1.0, 2.0, and 3.0. |
| Quality document v1.0 up-versioned in quick succession to v2.0, then v3.0. Only v3.0 enters the Effective (Steady) state. | The user deletes Quality document v2.0 before the connection job processes it in RIM. | RIM CrossLink v1.0 up-versioned to 3.0 in the Approved (Steady) state. Version history reflects versions 1.0 and 3.0. |
| Quality document v4.0 moved to the Obsolete state. | RIM CrossLink v4.0 exists, however the RIM Obsolete state type does not have a mapped state. | RIM CrossLink v4.0 remains in its current Approved (Steady) state. Vault generates a User Exception Message. |
| Quality document v3.1 Country field updated from “United States” only to “United States” and “Puerto Rico”. | Quality document v3.1 is in a draft state. | RIM CrossLink v3.0 Country field remains as “United States” only, until the Quality document enters a future Steady state (v4.0) and Vault updates the RIM CrossLink document and metadata. |
| Quality document v1.0’s Country field is empty (null) when the document enters the Effective (Steady) state. | The connection attempts to create RIM CrossLink v1.0 with an empty Country value. | Vault generates a User Exception Message. The Country field is required for this RIM document type. |
Product Transfer
The connection’s Product Transfer feature automatically transfers product data from a RIM Vault to a Quality Vault to provide a common set of data that can be referenced in Quality events and documents. The product data transferred includes:
- Product Family
- Product
- Product Variant
- Product Family Product
- Complex Product Component
This automation eliminates the overhead and potential user error associated with manually replicating product data. It also ensures that Vault applications remain consistent with regard to product data.
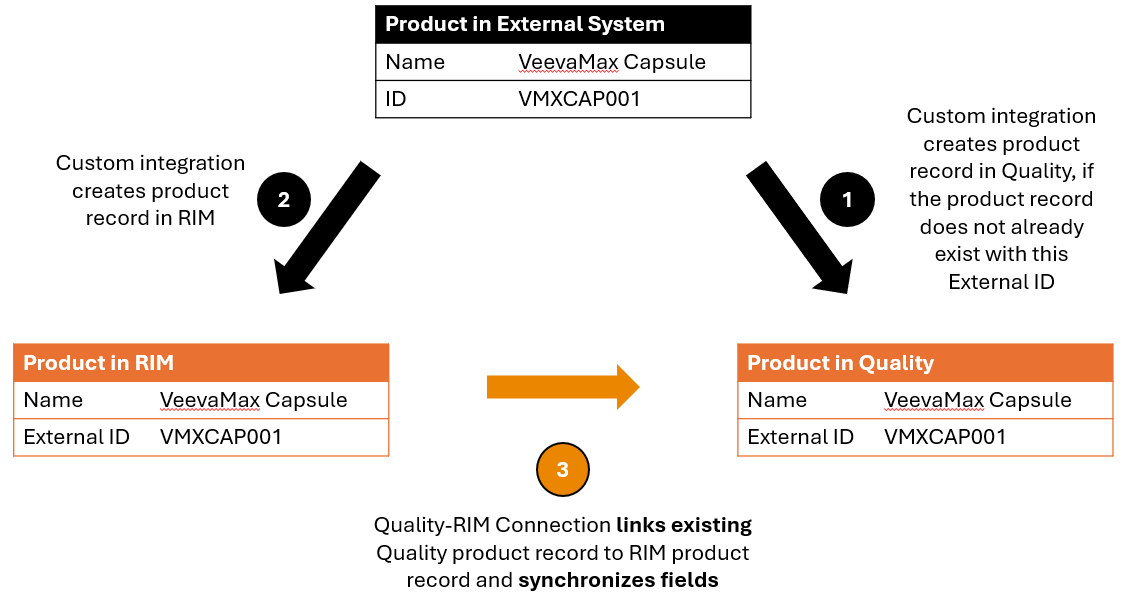
Master Product Data from External Systems
Some organizations manage master product data outside of Vault in an external system. They build custom integrations to directly push product data from the external system into their RIM and Quality Vaults. When this occurs, the connection’s Product Transfer feature relies upon an External ID field on Product Family, Product, and Product Variant records to ensure that data remains consistent across the two Vaults.
External integrations are responsible for assigning a unique identifier to product records created in the RIM and Quality Vaults. That way, when the connection’s Product Transfer feature runs, it knows whether a Product record in RIM matches a Product record in Quality. If the connection finds a match, it links the records in the two Vaults. Before creating a product record in the Quality Vault, an external integration must look for an existing record with a matching External ID to determine whether the connection already created it.
The diagram below illustrates how an organization’s external system manages master product data, integrates with the RIM and Quality Vaults, and uses the Quality-RIM Connection’s Product Transfer feature to keep the Vaults’ product data synchronized.